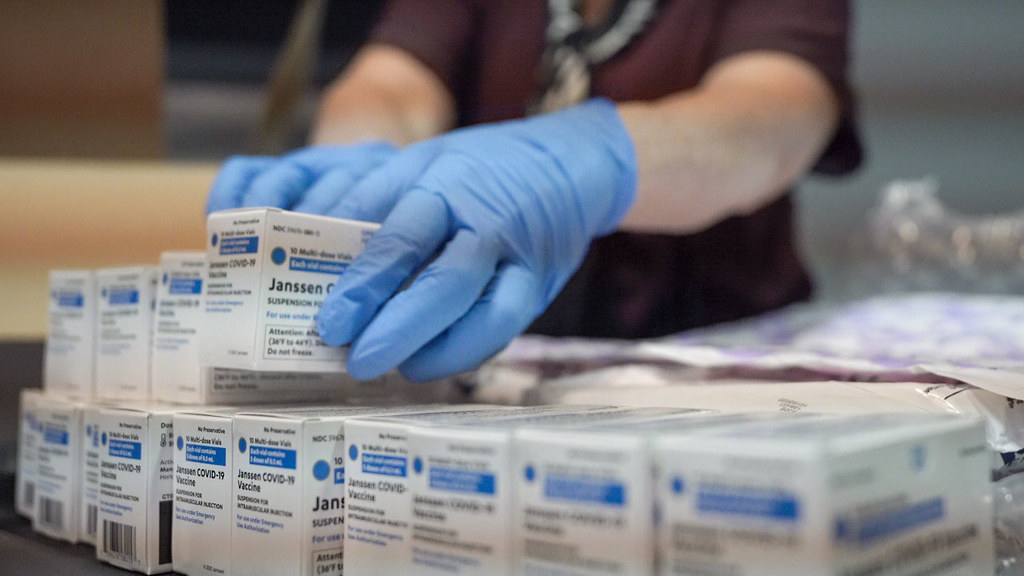
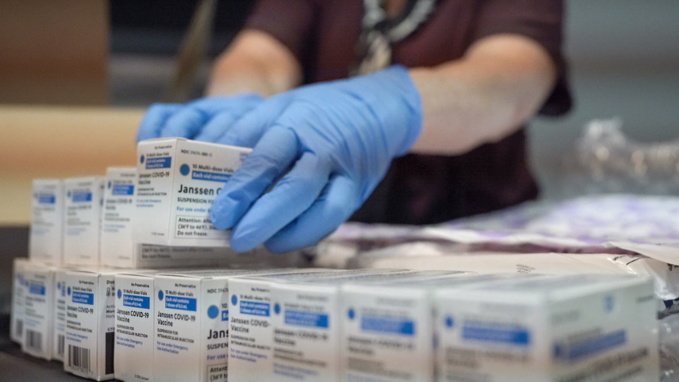
The CDC council advised that children this age be vaccinated with one of two vaccines: Moderna or Pfizer/BioNTech. The US Food and Drug Administration (FDA) had approved the vaccination of children under the age of five the day before.
"We have made another significant step forward in our country's fight against COVID-19 by working together and putting science first. We know that millions of parents and caregivers want to vaccinate their children, and now they can thanks to today's decision," Rochelle Walensky, Director of the Centers for Disease Control and Prevention, stated. Children's vaccinations should begin as early as next Tuesday. In the United States, there are around 20 million children of this age.
COVID-19 immunization for teenagers was licensed by US watchdogs in May of last year. Vaccination for children above the age of five was permitted later. The use of Pfizer's anti-covid booster for revaccination of children beyond the age of five was approved earlier this year.
source: cnn.com
"We have made another significant step forward in our country's fight against COVID-19 by working together and putting science first. We know that millions of parents and caregivers want to vaccinate their children, and now they can thanks to today's decision," Rochelle Walensky, Director of the Centers for Disease Control and Prevention, stated. Children's vaccinations should begin as early as next Tuesday. In the United States, there are around 20 million children of this age.
COVID-19 immunization for teenagers was licensed by US watchdogs in May of last year. Vaccination for children above the age of five was permitted later. The use of Pfizer's anti-covid booster for revaccination of children beyond the age of five was approved earlier this year.
source: cnn.com